The purpose of this video is to provide information on EPKINLY, epcoritamab-bysp, product handling and instructions for dilution.
Before we start it is important to understand the following.
INDICATIONS
EPKINLY is indicated for the treatment of adults with:
- relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS), including DLBCL arising from indolent lymphoma, and high-grade B-cell lymphoma (HGBCL) after 2 or more lines of systemic therapy.
- relapsed or refractory follicular lymphoma (FL) after 2 or more lines of systemic therapy.
These indications are approved under accelerated approval based on response rate and durability of response. Continued approval for these indications may be contingent upon verification and description of clinical benefit in confirmatory trials.
Please see the Important Safety Information, including Boxed Warnings for cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS), and information of other warnings and precautions at the end of this video.
The recommended dosage schedules differ based on indication; so please review the recommended dosage chapter before proceeding to the dose preparation sections.
The video is divided into eight chapters. Fastforward to the timestamps noted on the screen to review a specific chapter.
Chapter 1: Recommended Dosage and Storage & Handling
Your role in preparing EPKINLY is vital to patients and their care partners as key members of their care team.
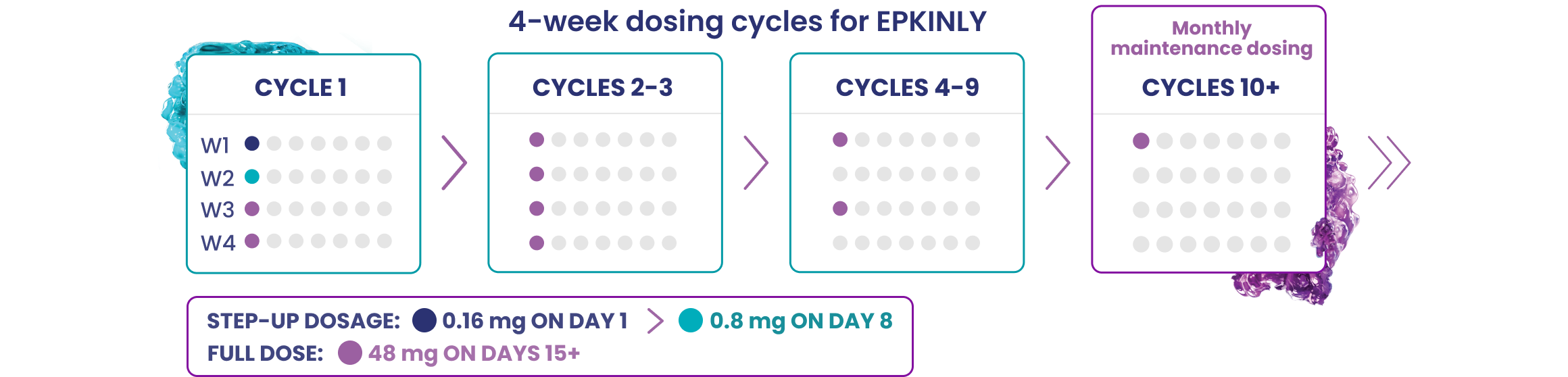
The recommended 2 step-up dosage schedule for EPKINLY in patients with DLBCL or HGBCL includes a 0.16 mg dose as step-up dose 1, a 0.8 mg dose as step-up dose 2, followed by a 48 mg full dose for subsequent doses.
EPKINLY is administered according to the following 28-day dosing cycles until disease progression or unacceptable toxicity.
The recommended 3 step-up dosage schedule for EPKINLY in patients with FL includes a 0.16 mg dose as step-up dose 1, a 0.8 mg dose as step-up dose 2, a 3 mg dose as step-up dose 3, followed by a 48 mg full dose for subsequent doses.
EPKINLY is administered according to the following 28-day dosing cycles until disease progression or unacceptable toxicity.
When planning the patient's treatment schedule, staff should verify availability of EPKINLY and recommended pre- and post- administration medications as detailed in the full Prescribing Information.
Herein, we will give detailed instructions on how to properly store and handle EPKINLY.
EPKINLY for subcutaneous injection is supplied in 2 single-dose vials. The 4 mg/0.8 mL single dose vial is used to prepare the step-up doses. The 48 mg/0.8 mL single-dose vial is used to prepare the full doses.
Storage of EPKINLY single-dose vials before preparation is as follows:
Store EPKINLY refrigerated at 2 to 8 degrees Celsius (36 to 46 degrees Fahrenheit)
Keep EPKINLY in the original carton to protect from light
After the preparation of EPKINLY for administration: Use EPKINLY solution in the syringe immediately
- If not used immediately, store the solution
- Refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours or
- At room temperature at 20°C to 25°C (68°F to 77°F) for up to 12 hours
- The total storage time from the start of dose preparation to administration should not exceed 24 hours
- Protect from direct sunlight
- Discard unused EPKINLY solution beyond the allowable storage time
Now that you know how to properly store and handle EPKINLY, let's go over how to properly prepare EPKINLY for administration in to your patients.
EPKINLY is for subcutaneous injection only.
Certain doses of EPKINLY require dilution prior to administration.
This video will provide step-by-step instructions for safely preparing EPKINLY using two available methods: an empty sterile vial method and a sterile syringe method.
Use aseptic technique to prepare EPKINLY. Filtration of the diluted solution is not required.
Follow the preparation instructions provided in this video and the full prescribing information, as improper preparation may lead to improper dose.
Chapter 2: 0.16 mg Dose (Step-up Dose 1) EMPTY STERILE VIAL METHOD
We will begin with the empty sterile vial method.
Here we will go through the necessary steps needed to prepare the EPKINLY 0.16 mg dose using the empty sterile vial method. Step-up dose 1 is the first dose administered to patients with DLBCL, HGBCL, or FL.
Let's begin by preparing your 0.16 mg dose dilution supplies.
Here is a compilation of all supplies you will need to assemble before you get started.
Now we will go through a simulation on how to prepare a 0.16 mg dose of EPKINLY using empty sterile vials.
Use aseptic technique to prepare EPKINLY.
Filtration of the diluted solution is not required.
To prepare the 0.16 mg dose, EPKINLY 4 mg/0.8 mL requires TWO dilutions.
When diluting EPKINLY, use an appropriately sized, syringe, vial, and needle for each transfer step.
First, retrieve one 4 mg/0.8 mL EPKINLY vial (with the turquoise cap) from the refrigerator.
Allow the vial to come to room temperature for no more than 1 hour.
In the meantime, grab your two appropriately sized empty sterile vials and label the first empty vial “Dilution A”.
And label your second empty vial “Dilution B”.
Grab the 4 mg/0.8 mL EPKINLY vial with the turquoise cap that is now at room temperature.
Gently swirl the EPKINLY vial.
DO NOT invert, vortex, or vigorously shake the vial.
Withdraw 0.8 mL of EPKINLY using an appropriately sized sterile syringe.
Transfer the 0.8 mL of EPKINLY into the Dilution A vial.
The EPKINLY vial is no longer needed and may be discarded.
REMINDER: Once you have pierced an empty vial, you have equalized the pressure within that vial, so for compounding practices, you need to make sure that you maintain negative pressure within the vial.
Grab one vial of 0.9% Sodium Chloride for injection. Withdraw 4.2 mL of 0.9% sodium chloride.
Transfer 4.2 mL of 0.9% sodium chloride into the Dilution A vial. The initially diluted solution contains 0.8 mg/ mL of EPKINLY.
Gently swirl the Dilution A vial for 30-45 seconds.
Next, grab the second empty vial labeled “Dilution B”. Withdraw 2 mL of solution from the Dilution A vial.
Transfer 2 mL of solution from the Dilution A vial into the Dilution B vial.
The Dilution A vial is no longer needed and can be discarded.
Grab your second vial of 0.9% sodium chloride for injection. Withdraw 8 mL of 0.9% sodium chloride.
Transfer 8 mL of 0.9% Sodium Chloride in to the Dilution B vial to make a final concentration of EPKINLY 0.16 mg/mL.
Gently swirl the Dilution B vial for 30-45 seconds.
Withdraw 1 mL of the diluted EPKINLY solution from the Dilution B vial into a syringe intended for subcutaneous injection in to the patient.
Label the syringe with the dose strength, 0.16 mg, and the time of day.
The syringe is now ready to be administered to the patient.
Refer to Storage & Handling instructions for EPKINLY solution in the syringe in Chapter 1.
REMINDER:
- EPKINLY is for subcutaneous injection ONLY
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit
- Each EPKINLY vial is a single-dose vial, meaning multiple doses must not be withdrawn from one vial
- After preparing each dose, discard the single-dose vial and any unused portion of EPKINLY
Chapter 3: 0.8 mg Dose (Step-up Dose 2) EMPTY STERILE VIAL METHOD
Here we will go through the necessary steps needed to prepare the EPKINLY 0.8 mg dose using the empty sterile vial method. Step-up dose 2 is the second dose administered to patients with DLBCL, HGBCL, or FL.
Let's begin by preparing your 0.8 mg dose dilution supplies.
Here is a compilation of all supplies you will need to assemble before you get started.
Now we will go through a simulation on how to prepare a 0.8 mg dose of EPKINLY using empty sterile vials.
Use aseptic technique to prepare EPKINLY.
Filtration of the diluted solution is not required.
To prepare the 0.8 mg dose, EPKINLY 4 mg/0.8 mL requires ONE dilution.
When diluting EPKINLY, use an appropriately sized syringe, vial, and needle for each transfer step.
First, retrieve one 4 mg/0.8 mL EPKINLY vial (with the turquoise cap) from the refrigerator.
Allow the vial to come to room temperature for no more than 1 hour.
Next, grab your appropriately sized empty sterile vial and label the empty vial “Dilution A”.
Grab the 4 mg/0.8 mL EPKINLY vial with the turquoise cap that is now at room temperature.
Gently swirl the EPKINLY vial.
DO NOT invert, vortex, or vigorously shake the vial.
Withdraw 0.8 mL of EPKINLY using an appropriately sized sterile syringe.
Transfer the 0.8 mL of EPKINLY into the Dilution A vial.
The EPKINLY vial is no longer needed and can be discarded.
REMINDER: Once you have pierced an empty vial, you have equalized the pressure within that vial, so for compounding practices, you need to make sure that you maintain negative pressure within this vial.
Grab your vial of 0.9% Sodium Chloride for injection.
Withdraw 4.2 mL of 0.9% Sodium Chloride.
Transfer 4.2 mL of 0.9% Sodium Chloride into the Dilution A vial to make a final concentration of EPKINLY 0.8 mg/mL.
Gently swirl the Dilution A vial for 30-45 seconds.
Withdraw 1 mL of the diluted EPKINLY solution from the Dilution A vial into a syringe intended for subcutaneous injection in to the patient.
Label the syringe with the dose strength, 0.8 mg, and the time of day.
The syringe is now ready to be administered to the patient.
Refer to Storage & Handling instructions for EPKINLY solution in the syringe in Chapter 1.
REMINDER:
- EPKINLY is for subcutaneous injection ONLY
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit
- Each EPKINLY vial is a single-dose vial, meaning multiple doses must not be withdrawn from one vial
- After preparing each dose, discard the single-dose vial and any unused portion of EPKINLY
Chapter 4: 0.16 mg Dose (Step-up Dose 1) STERILE SYRINGE METHOD
We will now review dilution using a sterile syringe method.
Here we will go through the necessary steps needed to prepare the EPKINLY 0.16 mg dose using the sterile syringe method. Step- up dose 1 is the first dose administered to patients with DLBCL, HGBCL, or FL.
Let’s begin by preparing your 0.16 mg dose dilution supplies.
Here is a compilation of all sterile supplies you will need to gather before you get started.
Now we will go through a simulation video on how to prepare a 0.16 mg dose of EPKINLY using the sterile syringe method.
Use aseptic technique to prepare EPKINLY. Filtration of the diluted solution is not required.
To prepare the 0.16 mg dose, EPKINLY 4 mg/0.8 mL requires TWO dilutions.
When diluting EPKINLY use an appropriately sized syringe, and needle for each transfer step.
First, retrieve one 4 mg/0.8 mL EPKINLY vial (with the turquoise cap) from the refrigerator.
Allow the vial to come to room temperature for no more than 1 hour.
Next, grab your 4 mg/0.8 mL EPKINLY vial with the turquoise cap that is now at room temperature.
Gently swirl the EPKINLY vial.
DO NOT invert, vortex, or vigorously shake the vial.
Retrieve four appropriately sized syringes. Label one “Dilution A”, and another “Dilution B”. Then, label another syringe “Syringe 1” and the last one “Syringe 2”.
Withdraw 0.8 mL of EPKINLY into Syringe 1.
Grab one vial of 0.9% Sodium Chloride for injection.
Withdraw 4.2 mL of 0.9% Sodium Chloride into the Dilution A syringe. Include approximately 0.2 mL of air in the syringe.
Connect Syringe 1 containing 0.8 mL of EPKINLY with the Dilution A syringe containing 0.9% Sodium Chloride. Push the 0.8 mL of EPKINLY into the Dilution A syringe. The initially diluted solution contains 0.8 mg/mL of EPKINLY.
Gently mix by inverting the connected syringes 180 degrees 5 times.
Disconnect the syringes and discard Syringe 1.
Connect Syringe 2 to the Dilution A syringe and transfer 2 mL of solution into Syringe 2.
The dilution A syringe is no longer needed.
Next, withdraw 8 mL of 0.9% Sodium Chloride into the Dilution B syringe. Include approximately 0.2 mL of air in the syringe.
Connect Syringe 2 to the Dilution B syringe. Push the 2 mL of EPKINLY solution into the Dilution B syringe to make a final concentration of 0.16 mg/mL.
Gently mix by inverting the connected syringes 180 degrees 5 times.
Disconnect the syringes and discard Syringe 2.
Connect the Dilution B syringe to a new syringe intended for administration in to the patient.
Withdraw 1 mL of the diluted EPKINLY solution from the Dilution B syringe into the new syringe intended for administration in to the patient.
The Dilution B syringe is no longer needed.
Label the syringe with the dose strength, 0.16 mg, and the time of day.
The syringe is now ready to be administered to the patient via subcutaneous injection.
Refer to Storage & Handling instructions for EPKINLY solution in the syringe in Chapter 1.
REMINDER:
- EPKINLY is for subcutaneous injection ONLY
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit
- Each EPKINLY vial is a single-dose vial, meaning multiple doses must not be withdrawn from one vial
- After preparing each dose, discard the single-dose vial and any unused portion of EPKINLY
Chapter 5: 0.8 mg Dose (Step-up Dose 2) STERILE SYRINGE METHOD
Here we will go through the necessary steps needed to prepare the EPKINLY 0.8 mg dose using the sterile syringe method. Step-up dose 2 is the second dose administered to patients with DLBCL, HGBCL, or FL.
Let’s begin by preparing your 0.8 mg dose dilution supplies.
Here is a compilation of all sterile supplies you will need to gather before you get started.
Now we will go through a simulation video on how to prepare a 0.8 mg dose of EPKINLY using the sterile syringe method.
Use aseptic technique to prepare EPKINLY.
Filtration of the diluted solution is not required.
To prepare the 0.8 mg dose, EPKINLY 4 mg/0.8 mL requires ONE dilution.
When diluting EPKINLY, use an appropriately sized syringe and needle for each transfer step.
First, retrieve one 4 mg/0.8 mL EPKINLY vial (with the turquoise cap) from the refrigerator.
Allow the vial to come to room temperature for no more than 1 hour.
Retrieve two appropriately sized syringes. Label one “Dilution A”, and another “Syringe 1”.
Grab the 4 mg/0.8 mL EPKINLY vial with the turquoise cap that is now at room temperature.
Gently swirl the EPKINLY vial.
DO NOT invert, vortex, or vigorously shake the vial.
Withdraw 0.8 mL of EPKINLY into Syringe 1.
Grab one vial of 0.9% Sodium Chloride for injection.
Withdraw 4.2 mL of 0.9% Sodium Chloride into the Dilution A syringe. Include approximately 0.2 mL of air in the syringe.
Connect Syringe 1 containing 0.8 mL of EPKINLY with the Dilution A syringe containing 0.9% Sodium Chloride. Push the 0.8 mL of EPKINLY in to the Dilution A syringe to make a final concentration of 0.8 mg/mL.
Gently mix by inverting the connected syringes 180 degrees 5 times.
Disconnect the syringes and discard Syringe 1.
Connect the syringe marked Dilution A to a new syringe intended for administration into the patient.
Withdraw 1 mL of the diluted EPKINLY solution from the Dilution A syringe into the new syringe intended for administration in to the patient.
The Dilution A syringe is no longer needed.
Label the syringe with the dose strength, 0.8 mg, and the time of day.
The syringe is now ready to be administered to the patient via subcutaneous injection.
Refer to Storage & Handling instructions for EPKINLY solution in the syringe in Chapter 1.
REMINDER:
- EPKINLY is for subcutaneous injection ONLY
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit
- Each EPKINLY vial is a single-dose vial, meaning multiple doses must not be withdrawn from one vial
- After preparing each dose, discard the single-dose vial and any unused portion of EPKINLY
Chapter 6: 3 mg Dose (Step-up Dose 3) FL ONLY
Now we will go through a simulation video on how to prepare a 3 mg dose of EPKINLY. EPKINLY 3 mg Step-up dose is required for FL patients only.
Please note that the 3 mg dose is prepared from a ready‑to‑use solution and does not require dilution.
Let's begin by preparing your 3 mg dose supplies.
Here is a compilation of all supplies you will need to assemble before you get started.
Here we will go through a simulation on how to prepare a 3 mg dose of EPKINLY.
Use aseptic technique to prepare EPKINLY.
First, retrieve one 4 mg/0.8 mL EPKINLY vial with the turquoise cap from the refrigerator.
Allow the vial to come to room temperature for no more than 1 hour.
Gently swirl the EPKINLY vial.
DO NOT invert, vortex, or vigorously shake the vial.
Withdraw 0.6 mL of EPKINLY into a syringe intended for subcutaneous injection in to the patient.
Label the syringe with the dose strength, 3 mg, and the time of day.
The syringe is now ready to be administered to the patient.
Refer to Storage & Handling instructions for EPKINLY solution in the syringe in Chapter 1.
REMINDER:
- EPKINLY is for subcutaneous injection ONLY
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit
- Each EPKINLY vial is a single-dose vial, meaning multiple doses must not be withdrawn from one vial
- After preparing each dose, discard the single-dose vial and any unused portion of EPKINLY
Chapter 7: 48 mg Dose (Full Dose)
Lastly, we will go through the necessary steps needed to prepare the EPKINLY 48 mg dose. This is the full dose administered to patients with DLBCL, HGBCL, or FL.
Please note that the 48 mg dose is prepared from a ready-to-use solution and does not require dilution.
Let's begin by preparing your 48 mg dose supplies.
Here is a compilation of all supplies you will need to assemble before you get started.
Here we will go through a simulation on how to prepare a 48 mg dose of EPKINLY.
Use aseptic technique to prepare EPKINLY.
First, retrieve the 48 mg/0.8 mL EPKINLY vial with the orange cap from the refrigerator.
Allow the vial to come to room temperature for no more than 1 hour.
Gently swirl the EPKINLY vial.
DO NOT invert, vortex, or vigorously shake the vial.
Withdraw 0.8 mL of EPKINLY into a syringe intended for subcutaneous injection in to the patient.
Label the syringe with the dose strength, 48 mg, and the time of day.
The syringe is now ready to be administered to the patient.
Refer to Storage & Handling instructions for EPKINLY solution in the syringe in Chapter 1.
REMINDER:
- EPKINLY is for subcutaneous injection ONLY
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit
- Each EPKINLY vial is a single-dose vial, meaning multiple doses must not be withdrawn from one vial
- After preparing each dose, discard the single-dose vial and any unused portion of EPKINLY
Chapter 8: Important Safety Information for EPKINLY® (epcoritamab-bysp)
The following is the important safety information for EPKINLY.
BOXED WARNINGS
- Cytokine release syndrome (CRS), including serious or life-threatening reactions, can occur in patients receiving EPKINLY. Initiate treatment with the EPKINLY step-up dosage schedule to reduce the incidence and severity of CRS. Withhold EPKINLY until CRS resolves or permanently discontinue based on severity
- Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), including life-threatening and fatal reactions, can occur with EPKINLY. Monitor patients for neurological signs or symptoms of ICANS during treatment. Withhold EPKINLY until ICANS resolves or permanently discontinue based on severity
CRS
- CRS occurred in 51% of patients with large B-cell lymphoma (LBCL) in the clinical trial (37% grade 1, 17% grade 2, and 2.5% grade 3) and recurred in 16% of patients. Most events (92%) occurred during cycle 1, with 61% occurring after the 48 mg dose on cycle 1, day 15.
- CRS occurred in 49% of patients with FL receiving the recommended 3 step up dosage schedule in the clinical trial (45% grade 1, 9% grade 2) and recurred in 23% of patients. Most events (88%) occurred during cycle 1, with 49% occurring after the 48 mg dose on cycle 1, day 22.
- In patients who experienced CRS, the signs and symptoms included pyrexia, hypotension, hypoxia, dyspnea, chills, and tachycardia. Concurrent neurological adverse reactions associated with CRS occurred in 2.5% of patients with LBCL (reactions included headache, confusional state, tremors, dizziness, and ataxia) and 4.7% of patients with FL (reactions included headache and dizziness).
- Administer pretreatment medications to reduce the risk of CRS.
- Patients with DLBCL or high-grade B-cell lymphoma should be hospitalized for 24 hours following administration of the first full 48 mg dose.
- Monitor patients for potential CRS. At the first signs or symptoms of CRS, manage per current practice guidelines and administer supportive care as appropriate.
ICANS
- ICANS occurred in 6% of patients with LBCL in the clinical trial (4.5% grade 1, 1.3% grade 2, 0.6% fatal). Of the 10 ICANS events, 9 occurred in cycle 1 of treatment.
- ICANS occurred in 6% of patients with FL receiving the 2-step up dosage schedule in the clinical trial (3.9% grade 1, 2.4% grade 2).
- The onset of ICANS can be concurrent with CRS, following resolution of CRS, or in the absence of CRS. Clinical manifestations of ICANS included, but were not limited to, confusional state, lethargy, tremor, dysgraphia, aphasia, and non-convulsive status epilepticus.
- Monitor patients for potential ICANS. At the first signs or symptoms of ICANS, manage per current practice guidelines and administer supportive care as appropriate.
Infections
- EPKINLY can cause serious and fatal infections. Serious infections, including opportunistic infections, were reported in 15% of patients with LBCL in the clinical trial (most common: 4.5% sepsis, 3.2% pneumonia). Fatal infections occurred in 1.3% of patients (1.3% COVID-19).
- Serious infections, including opportunistic infections, were reported in 40% of patients with FL receiving the 2-step up dosage schedule in the clinical trial (most common: 20% COVID-19, 13% pneumonia, 3% urinary tract infections). Fatal infections occurred in 6% of patients (5% COVID-19, 0.8% pneumonia, 0.8% sepsis).
- Monitor patients for signs and symptoms of infection prior to and during treatment and treat appropriately. Avoid administration in patients with active infections. Withhold or consider permanent discontinuation of EPKINLY based on severity. Prior to starting EPKINLY, provide Pneumocystis jirovecii pneumonia (PJP) prophylaxis and consider prophylaxis against herpes virus.
Cytopenias
- EPKINLY can cause serious or severe cytopenias. In the clinical trial of patients with LBCL, grade 3 or 4 events occurred in 32% (neutrophils decreased), 12% (hemoglobin decreased), and 12% (platelets decreased). Febrile neutropenia occurred in 2.5%.
- In the clinical trial of patients with FL receiving the 2-step up dosage schedule, grade 3 or 4 events occurred in 30% (neutrophils decreased), 10% (hemoglobin decreased), and 8% (platelets decreased). Febrile neutropenia occurred in 3.1%.
- Monitor complete blood counts throughout treatment. Based on severity of cytopenias, temporarily withhold or permanently discontinue EPKINLY. Consider prophylactic granulocyte colony-stimulating factor administration as applicable.
Embryo-Fetal Toxicity
- EPKINLY may cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with EPKINLY and for 4 months after the last dose. Verify pregnancy status in females of reproductive potential prior to initiating EPKINLY.
Adverse Reactions
- DLBCL/HGBCL: Most common (≥20%) adverse reactions were CRS, fatigue, musculoskeletal pain, injection site reactions, pyrexia, abdominal pain, nausea, and diarrhea. Most common grade 3 to 4 laboratory abnormalities (≥10%) were decreased lymphocytes, decreased neutrophils, decreased white blood cells, decreased hemoglobin, and decreased platelets.
- FL: Most common (≥20%) adverse reactions were injection site reactions, CRS, COVID-19, fatigue, upper respiratory tract infection, musculoskeletal pain, rash, diarrhea, pyrexia, cough, and headache. The most common grade 3 to 4 laboratory abnormalities (≥10%) were decreased lymphocytes, decreased neutrophils, decreased white blood cells, and decreased hemoglobin.
Use in Specific Populations
- Lactation: Advise women not to breastfeed during treatment and for 4 months after the last dose of EPKINLY.
- Geriatric Use: In patients with relapsed or refractory FL who received EPKINLY in the clinical trial, 52% were ≥65 years old, and 13% were ≥75 years old. A higher rate of fatal adverse reactions, primarily infections, including COVID-19, was observed in patients ≥65 years old compared to younger adult patients. No overall difference in efficacy was observed.
Please see link above for Full Prescribing Information.