
Even with a high enrollment of patients with difficult-to-treat disease,* 60% CR was observed in EPCORE® NHL-11-4
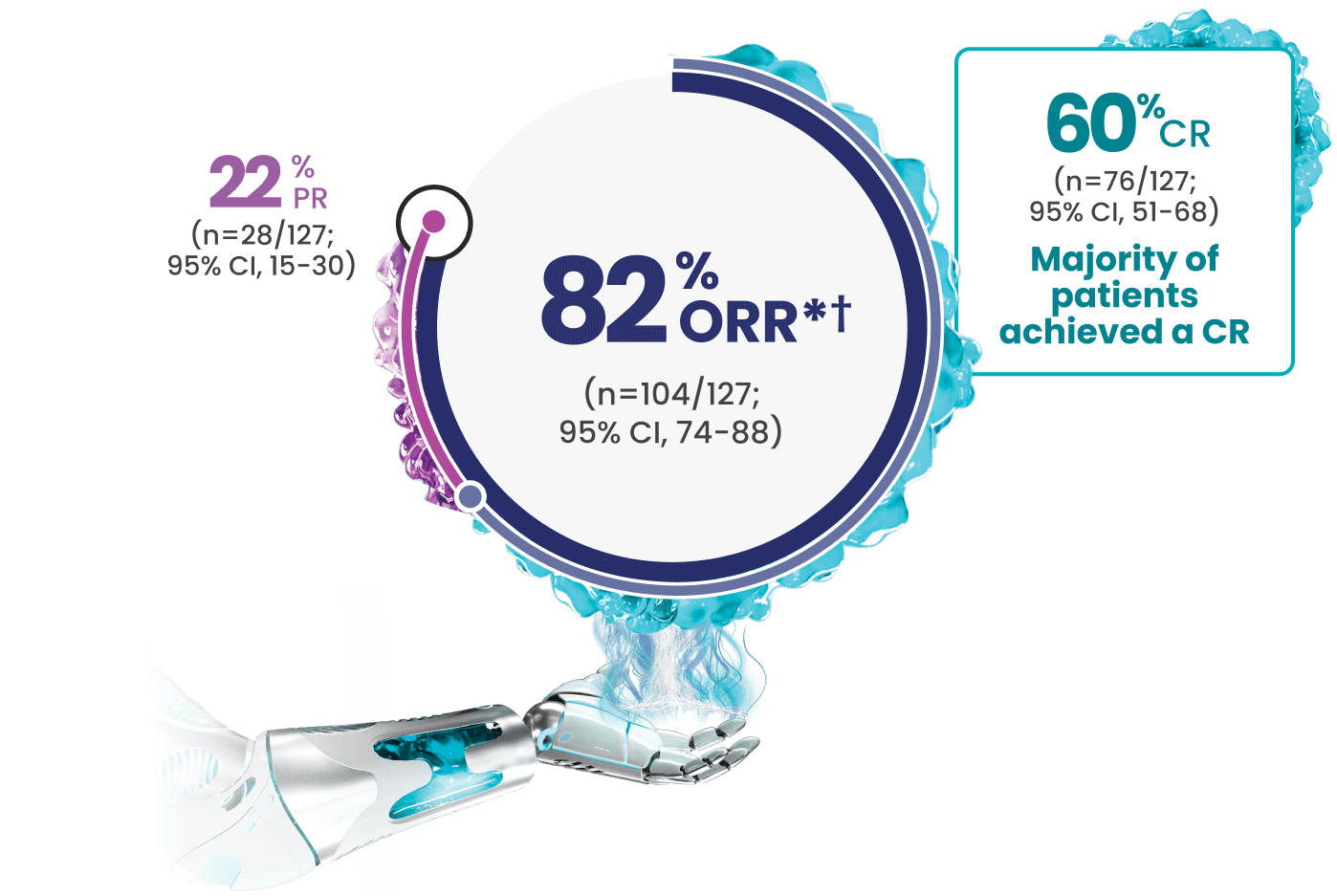
*Difficult-to-treat disease characteristics included age >65, double refractory, refractory to ≥2 consecutive lines of antilymphoma therapy, and POD24.1-4
†Efficacy results determined by Lugano criteria (2014) as assessed by Independent Review Committee (IRC).1
‡Median study follow-up was 17.4 months.6
Rapid response1
- In responders (n=104), the median time to first response was 1.4 months (range: 1-3 months)†
In a prespecified analysis of complete responders (n=76), the median time to complete response was 1.5 months (range: 1.2-11.1).5†
*Difficult-to-treat disease characteristics included age >65, double refractory, refractory to ≥2 consecutive lines of antilymphoma therapy, and POD24.1-4
†Efficacy results determined by Lugano criteria (2014) as assessed by Independent Review Committee (IRC).1
‡Median study follow-up was 17.4 months.6
The efficacy results of the 86 patients who received the recommended 3-step up dosage schedule in the EPCORE® NHL-1 dose optimization cohort were comparable to the primary efficacy population.1§
- Investigator-assessed efficacy outcomes6,7:
- ORR: 86% (n=74/86; 95% CI, 76.9-92.6)
- CR: 64% (n=55/86; 95% CI, 52.9-74.0)
- PR: 22% (n=19/86)
§Median study follow-up for the dose optimization cohort was 5.7 months.6
Responses demonstrated in select refractory subgroups1,5
Double refractory
![Double Refractory Response: yes (75 percent ORR [n equals 67 out of 89; 95 percent CI, 65 to 84], 55 percent CR [n equals 49], and 20% PR [n equals 18]) and no (97 percent ORR [n equals 37 out of 38; 95 percent CI, 86 to 100], 71 percent CR [n equals 27], and 26 percent PR [n equals 10]).](/content/dam/epcoritamabhcp/images/clinical-trial-results/responses-in-double-refactory-desktop.png)
Refractory to last therapy
![Refractory to last therapy responses: yes (74 percent ORR [n equals 65 out of 88; 95 percent CI, 63 to 83], 49 percent CR [n equals 43], and 25 percent PR [n equals 22]) and no (100 percent ORR [n equals 39 out of 39]; 95 percent CI, 91 to 100), 85 percent CR [n equals 33], and 15 percent PR [n equals 6]).](/content/dam/epcoritamabhcp/images/clinical-trial-results/responses-in-last-therapy-refactory-desktop.png)
Data Limitation: Study was not powered to evaluate these prespecified subgroups. Data are exploratory and descriptive in nature. No formal inferences can be drawn.
- Double refractory: Patients were refractory to both anti-CD20 and an alkylating agent6
- Refractory: Patient with no response or relapse within 6 months after completing therapy6
ORR, CR, and PR rates observed across additional subgroups5
![Response rates demonstrated in select additional subgroups. Patient response percentages for age in years: less than 65 years (n equals 61) (28 percent PR, 55 percent CR, 84 percent ORR [95 percent CI: 72 to 92]) and greater than or equal to 65 years (n equals 66) (16 percent PR, 64 percent CR, and 80 percent ORR [95 percent CI: 69 to 89]). For number of prior lines of treatment: 2 prior lines (n equals 46) (19 percent PF, 70 percent CR, and 89 percent ORR [95 percent Cl: 76 to 96]), 3 prior lines (n equals 41) (25 percent PR, 63 percent CR, and 88 percent ORR [95 percent Cl: 74 to 96]), and 4 or more prior lines (n equals 40) (23 percent PR, 45 percent CR, and 68 percent ORR [95 percent Cl: 51 to 81]). For progression within 2 years of any 1L therapy: Yes (n equals 66) (17 percent PR, 62 percent CR, and 79 percent ORR [95 percent CI: 67 to 88]) and No (n equals 61) (28 percent PR, 57 percent CR, and 85 percent ORR [95 percent CI, 74 to 93]).](/content/dam/epcoritamabhcp/images/clinical-trial-results/observed-across-additional-subgroups-desktop.png)
Data Limitation: Study was not powered to evaluate these prespecified subgroups. Data are exploratory and descriptive in nature. No formal inferences can be drawn.
EPKINLY delivered durable responses with mDOR not reached1,5†||
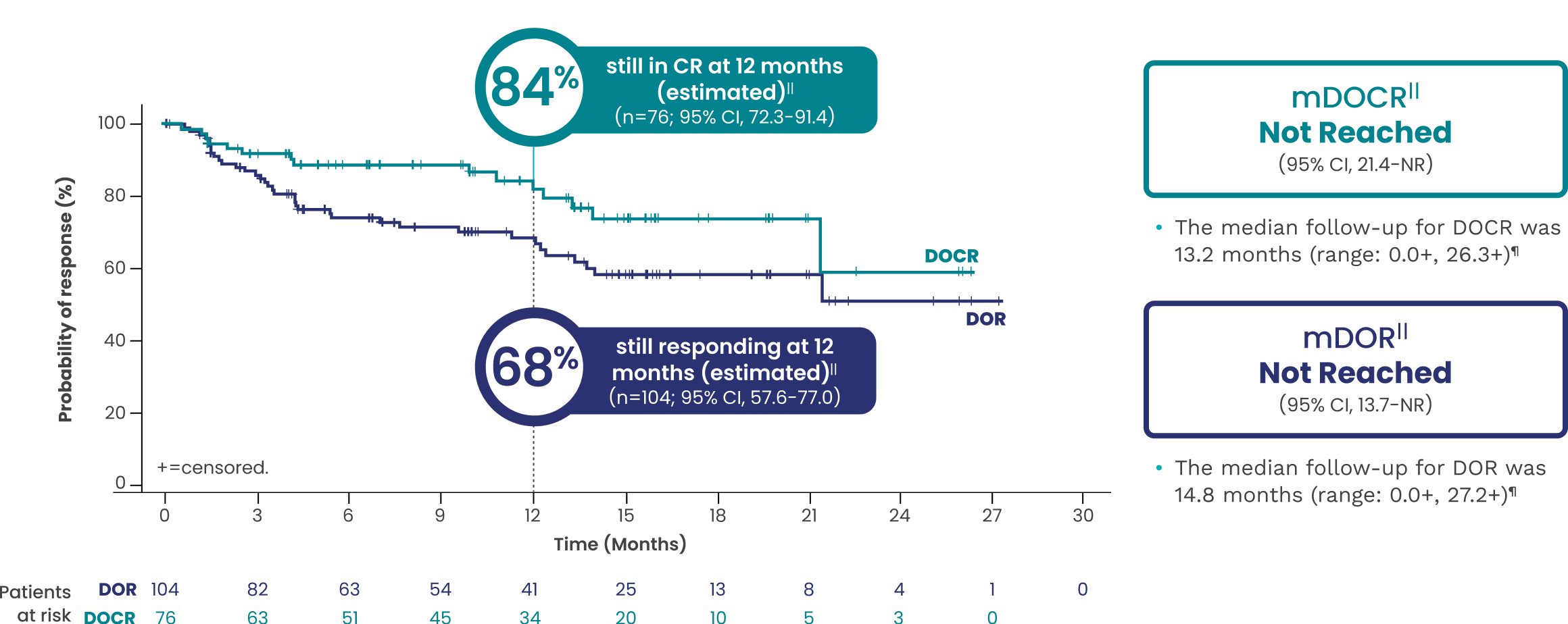
Date of analysis: April 2023.
†Efficacy results determined by Lugano criteria (2014) as assessed by Independent Review Committee (IRC).1
||Based on Kaplan-Meier estimate.
¶Both lower and upper limits of the range indicate a censored value.
3L=third line; CI=confidence interval; CR=complete response; DOCR=duration of complete response; DOR=duration of response; FL=follicular lymphoma; mDOCR=median duration of complete response; mDOR=median duration of response; NHL=non-Hodgkin Lymphoma; NR=not reached; ORR=overall response rate; POD24=progression of disease within 24 months; PR=partial response.



