
EPKINLY: Broadest eligibility of any bispecific antibody in 3L+ FL1-4
- The EPCORE® NHL-1 trial allowed for the inclusion of patients with ECOG PS 2, absolute neutrophil count ≥1 x 10⁹/L, creatinine clearance ≥45 mL/min, any hemoglobin level when starting EPKINLY, and significant pulmonary disease
An open-label, multicohort, multicenter, single-arm trial that included patients with R/R FL after 2 or more lines of systemic therapy
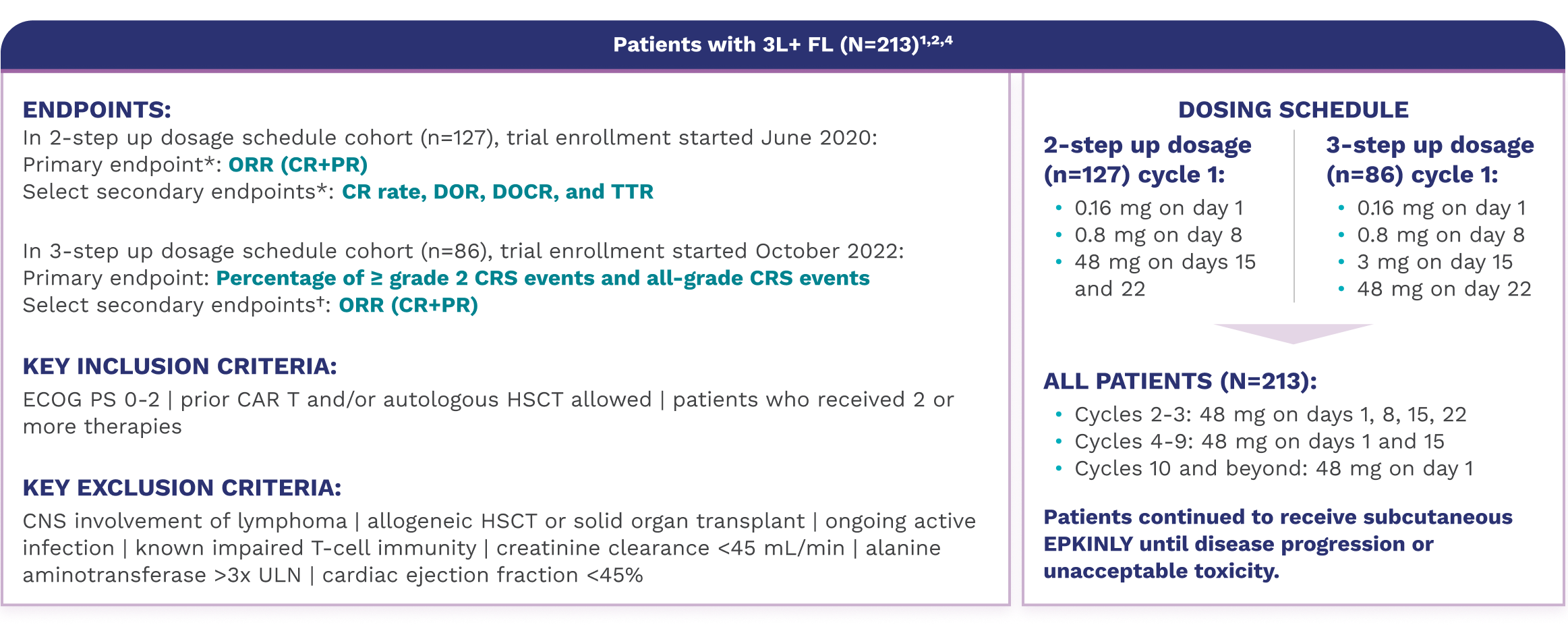
The 3-step up dosage schedule is the recommended dosage for patients with 3L+ FL. A separate dose optimization cohort evaluated the recommended 3-step up dosage schedule for CRS mitigation. See the recommended 3-step up dosage schedule for 3L+ patients with FL.
*Efficacy results determined by Lugano criteria (2014) as assessed by Independent Review Committee (IRC).
†Assessed by investigator (INV).
EPKINLY was studied in a population that included difficult-to-treat‡ patients with R/R FL1,5-7
EPKINLY was evaluated in patients with 3L+ FL with characteristics linked to a poor prognosis
Select patient characteristics
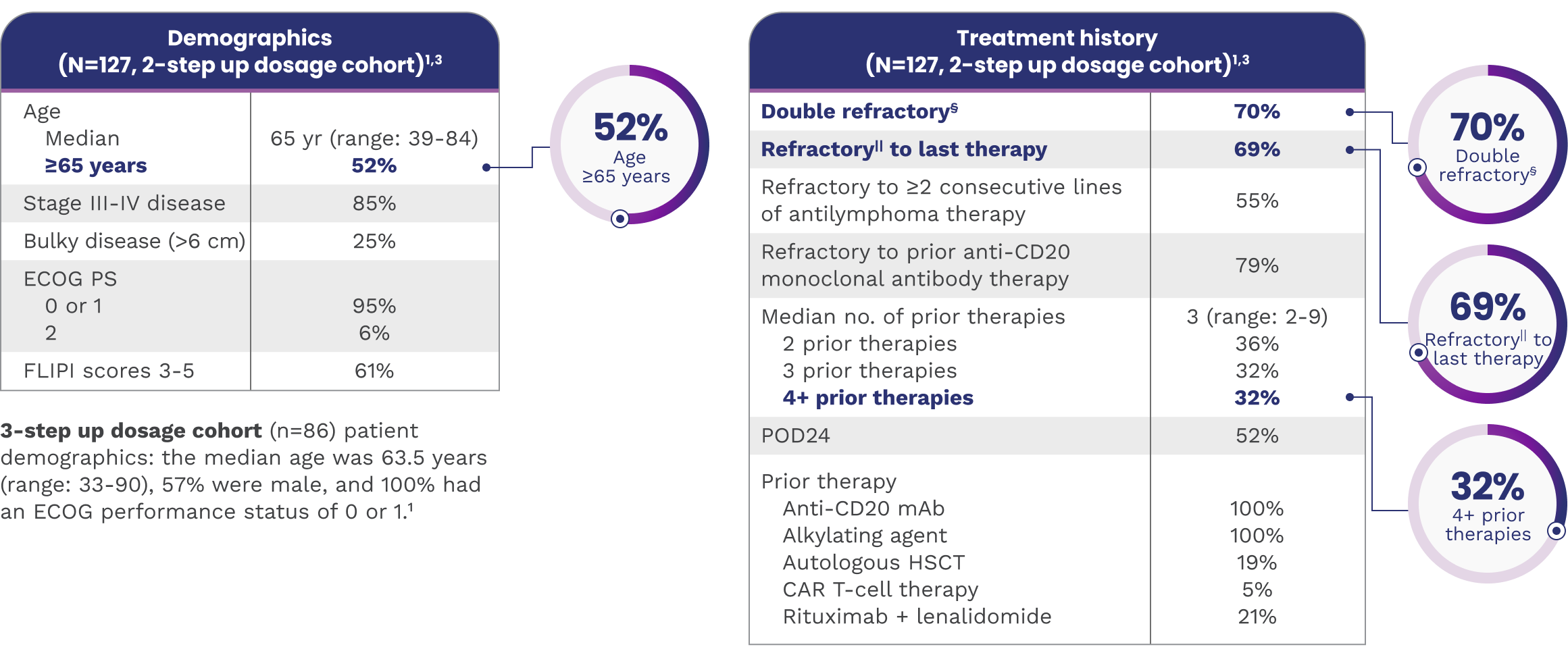
‡Difficult-to-treat disease characteristics included age >65, double refractory, refractory to ≥2 consecutive lines of antilymphoma therapy, and POD24.1,5-7
§Defined as refractory to both anti-CD20 monoclonal antibody and alkylator therapy.4
||Refractory: Patient with no response or relapse within 6 months after therapy.4
3L=third line; CAR T=chimeric antigen T-cell therapy; CD20=cluster of differentiation 20; CNS=central nervous system; CR=complete response; CRS=cytokine release syndrome; DOCR=duration of complete response; DOR=duration of response; ECOG PS=Eastern Cooperative Oncology Group performance status; FL=follicular lymphoma; FLIPI=Follicular Lymphoma International Prognostic Index; HSCT=hematopoietic stem cell transplant; mAb=monoclonal antibody; mg=milligram; min=minute; mL=milliliter; NHL=non-Hodgkin lymphoma; ORR=overall response rate; PK=pharmacokinetics; POD24=progression of disease within 24 months; PR=partial response; R/R=relapsed/refractory; TTR=time to response; ULN=upper limit of normal.



