
CRS was primarily low grade, predictable, and
manageable1
In patients who received EPKINLY at the recommended 3-step up dosage schedule (n=86)
in the EPCORE® NHL-1 trial
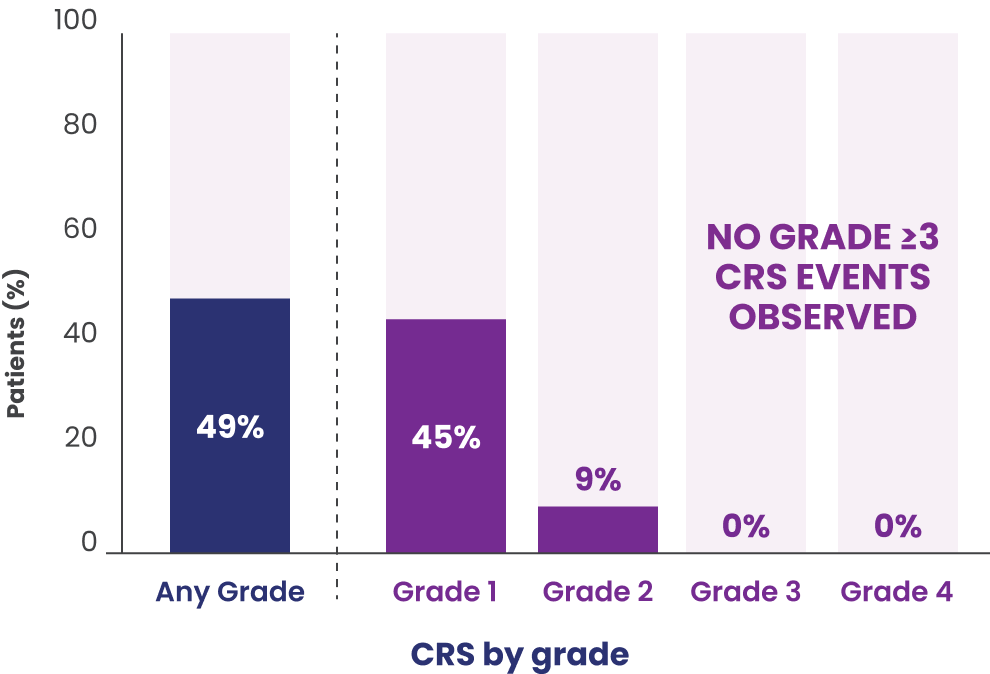
- Recurrent CRS occurred in 23% of patients
- Most CRS events (88%) occurred during cycle 1
- In cycle 1, 14% occurred after the 0.16-mg dose (day 1), 7% after the 0.8-mg dose (day 8), 17% after the 3-mg dose (day 15), and 49% after the 48-mg dose (day 22)
- The median time to onset of CRS from the most recently administered EPKINLY dose across all doses was 59 hours (range: 0.1-7 days)
- The median time to onset after the first full 48-mg dose was 61 hours (range: 0.1-7 days)
- CRS resolved in 100% of patients
- Median duration of CRS events was 2 days (range: 1-14 days)
Monitor patients for potential CRS. At the first signs or symptoms of CRS, immediately evaluate patients for hospitalization, manage per current practice guidelines, and administer supportive care as appropriate. Withhold or discontinue EPKINLY as recommended. See CRS Management.
SELECT IMPORTANT SAFETY INFORMATION
Cytokine release syndrome (CRS), including serious or life-threatening reactions, can occur in patients receiving EPKINLY. Initiate treatment with the EPKINLY step-up dosage schedule to reduce the incidence and severity of CRS. Withhold EPKINLY until CRS resolves or permanently discontinue based on severity.
- In patients who experienced CRS, the signs and symptoms included pyrexia, hypotension, hypoxia, dyspnea, chills, and tachycardia. Concurrent neurological adverse reactions associated with CRS occurred in 4.7% of patients with FL and included headache and dizziness.
- Administer pretreatment medications to reduce risk of CRS.
ICANS events in EPCORE NHL-1 trial in 3L+ FL patients1,2
In patients with FL who received EPKINLY following the 2-step up dosage schedule (n=127)1:
- ICANS events occurred in 6% (8/127) of patients with FL receiving EPKINLY, utilizing the 2-step up dosage schedule
- 3.9% grade 1, 2.4% grade 2
- The median time to onset of ICANS was 21.5 days (range: 14-66 days) from the start of treatment
- Relative to the most recent administration, the median time to onset was 3 days (range: 0.4-7 days)
- ICANS resolved in 100% of patients
- The median duration of ICANS was 2 days (range: 1-7 days)
- The median duration of exposure for patients was 8 cycles (range: 1-33 cycles)
In patients who received EPKINLY following the recommended 3-step up dosage schedule (n=86)1,2:
- No ICANS events were observed at the time of analysis. The median exposure for patients in the dose optimization cohort was 5 cycles (range: 1-12 cycles). No conclusions regarding the rate of ICANS can be made, as exposure may not be sufficient in this cohort.
Monitor patients for potential ICANS following EPKINLY. At the first signs or symptoms of ICANS, immediately evaluate patient and provide supportive therapy based on severity. Withhold or discontinue EPKINLY per recommendations and consider further management per current practice guidelines. See ICANS Management.
SELECT IMPORTANT SAFETY INFORMATION
Immune effector cell-associated neurotoxicity syndrome (ICANS), including life-threatening and fatal reactions, can occur with EPKINLY. Monitor patients for neurological signs or symptoms of ICANS during treatment. Withhold EPKINLY until ICANS resolves or permanently discontinue based on severity.
- The onset of ICANS can be concurrent with CRS, following resolution of CRS, or in the absence of CRS. Clinical manifestations of ICANS included, but were not limited to, confusional state, lethargy, tremor, dysgraphia, aphasia, and non-convulsive status epilepticus.
3L=third line; CRS=cytokine release syndrome; FL=follicular lymphoma; ICANS=immune effector cell-associated neurotoxicity; mg=milligram; NHL=non-Hodgkin lymphoma.





